Churg Strauss Syndrome: What is Churg Strauss Syndrome?
Churg Strauss Syndrome is a rare autoimmune disorder marked by eosinophils, a specific type of white blood cell. It impacts different parts of the body, causing a variety of symptoms. Grasping the causes, symptoms, and treatment options is vital for managing this complex condition. Those affected face significant health challenges. Timely diagnosis and proper care are critical.
Healthcare professionals can develop effective treatment strategies by understanding the underlying factors and manifestations of this syndrome. This approach aims to enhance patient outcomes. To grasp Churg Strauss Syndrome, we must explore its historical roots and medical categorization. Now known as Eosinophilic Granulomatosis with Polyangiitis (EGPA), it has long fascinated medical professionals. This is due to its complex nature and the hurdles in diagnosing and treating it.
Historical Background and Discovery
In 1951, Jacob Churg and Lotte Strauss first documented Churg Strauss Syndrome. They noted a subset of patients with asthma and eosinophilia suffering from severe vasculitis. The historical context of its discovery is key to understanding its evolution in medical perception and classification.
- Initially identified as a distinct form of vasculitis
- Associated with asthma and eosinophilia
- Recognized as a rare condition with significant morbidity
Classification as a Vasculitis
Churg Strauss Syndrome is categorized as a vasculitis, affecting small to medium-sized blood vessels. This classification is vital for grasping its pathophysiology and guiding treatment strategies.
Being classified as a vasculitis indicates inflammation of blood vessels. This inflammation can manifest in various ways, depending on the affected vessels and organs.
- Vasculitis involves inflammation of blood vessels
- Can affect various organs and systems
- Treatment often involves immunosuppression
Understanding Eosinophilic Granulomatosis with Polyangiitis
The term Eosinophilic Granulomatosis with Polyangiitis (EGPA) now accurately describes a specific vasculitis type, previously known as Churg Strauss Syndrome. This name change reflects a deeper understanding of its pathophysiology and its role in vasculitic disorders.
Modern Terminology and Classification
EGPA’s modern classification is based on its distinctive features. These include eosinophilia, granulomatous inflammation, and vasculitis affecting various organs. This classification is key for diagnosing and managing the condition effectively.
EGPA falls under the category of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides. Yet, not all EGPA patients test positive for ANCA. This classification aids in understanding the disease’s pathogenesis and guiding treatment.
Relationship to Other Vasculitides
EGPA is among several vasculitides that must be differentiated based on their clinical and pathological features. Grasping its relationship to other vasculitides is vital for accurate diagnosis and treatment.
Comparison with Granulomatosis with Polyangiitis
EGPA and Granulomatosis with Polyangiitis (GPA) share some similarities, both being ANCA-associated vasculitides. Yet, EGPA is marked by its significant eosinophilia and asthma. While both can affect multiple organs, eosinophilia is a defining feature of EGPA.
Comparison with Microscopic Polyangiitis
Microscopic Polyangiitis (MPA) is another vasculitis type that must be distinguished from EGPA. MPA lacks granulomatous inflammation and eosinophilia, unlike EGPA. The clinical presentation and ANCA status help differentiate between these two conditions.
Epidemiology of Churg Strauss Syndrome
Churg Strauss Syndrome, now more commonly referred to as Eosinophilic Granulomatosis with Polyangiitis (EGPA), presents a complex epidemiological profile. This complexity necessitates a thorough examination. Understanding its prevalence, incidence, and demographic characteristics is essential.
Prevalence and Incidence Rates
The prevalence of EGPA is estimated to be between 10.7 and 14.4 per million adults. Its annual incidence ranges from 0.5 to 6.8 per million.
- Prevalence rates vary significantly across different populations.
- Incidence rates have been observed to fluctuate, potentially due to changes in diagnostic criteria and awareness.

Demographic Patterns
Demographic patterns in EGPA reveal interesting trends in age, gender, and geographic distribution.
Age and Gender Distribution
EGPA can occur at any age, but it typically manifests between the ages of 30 and 50. There is a slight male predominance in some studies.
- The condition is rare in children.
- Adults in their fourth to fifth decade of life are most commonly affected.
Geographic Variations
Geographic variations in the incidence and prevalence of EGPA have been observed. These variations may be due to genetic, environmental, or healthcare access factors.
- Some studies suggest a higher incidence in certain European populations.
- The condition’s rarity makes global epidemiological comparisons challenging.
Pathophysiology and Etiology
Churg Strauss Syndrome is a complex condition, influenced by autoimmune mechanisms, genetic predisposition, and environmental factors. These elements interact, resulting in the diverse symptoms seen in patients. Understanding these factors is key to grasping the syndrome’s nature.
Autoimmune Mechanisms
The autoimmune aspect of Churg Strauss Syndrome is marked by the presence of anti-neutrophil cytoplasmic antibodies (ANCA) in many patients. These autoantibodies are vital in the disease’s progression, activating neutrophils and causing vascular inflammation.
The syndrome also involves an immune system imbalance, with an overactive eosinophil response. Eosinophils are central to the disease, causing tissue damage and organ dysfunction.
Genetic Predisposition
Genetic predisposition is thought to increase the risk of developing Churg Strauss Syndrome. Specific genetic variations may lead to an abnormal immune response, potentially triggering the syndrome.
Environmental Triggers
Environmental factors, like allergens and certain medications, can trigger Churg Strauss Syndrome in susceptible individuals.
Medication-Induced Cases
Some medications, like leukotriene modifiers for asthma, have been linked to Churg Strauss Syndrome. The exact cause is unclear, but these drugs may reveal underlying conditions or shift the immune response.
Allergen Exposure
Allergen exposure is another environmental trigger. Patients with allergies or asthma are more prone to developing Churg Strauss Syndrome. This suggests that allergens may play a role in the disease’s development.
Clinical Phases of Churg Strauss Syndrome
Churg Strauss Syndrome is divided into three clinical phases, each with distinct features. Recognizing these phases is key to diagnosing and managing the condition properly.
Prodromal (Allergic) Phase
The prodromal phase is the first stage, marked by allergic symptoms like asthma and allergic rhinitis. These symptoms can appear years before the other phases.
- Allergic rhinitis
- Asthma
- Recurrent sinusitis
Eosinophilic Phase
The eosinophilic phase comes next, characterized by high eosinophil counts and their infiltration into organs like the lungs and gastrointestinal tract.
- Peripheral eosinophilia
- Eosinophilic infiltration of organs
- Gastrointestinal symptoms
Vasculitic Phase
The vasculitic phase is the most severe, featuring vasculitis in multiple organs. This phase can cause severe illness if not treated quickly.
- Vasculitis affecting multiple organs
- Cardiac involvement
- Neurological manifestations
Patients progress through these phases differently, and not all may show all three. Yet, grasping these phases is vital for early diagnosis and effective treatment of Churg Strauss Syndrome.
Common Symptoms and Clinical Manifestations
Churg Strauss Syndrome is marked by a complex array of symptoms. These include respiratory, dermatological, and neurological manifestations. The condition’s symptoms vary widely among patients.
Respiratory Symptoms
Respiratory symptoms are a hallmark of Churg Strauss Syndrome. Patients often experience severe asthma, which can be challenging to manage with conventional treatments.
Asthma and Pulmonary Infiltrates
Asthma is a common presenting feature, often accompanied by pulmonary infiltrates visible on chest radiographs. These infiltrates can be transient or persistent.
Sinusitis and Nasal Polyps
Sinusitis and nasal polyps are also frequent, contributing to the respiratory morbidity associated with the syndrome. Chronic sinusitis can lead to significant discomfort and complications.

Skin Manifestations
Skin manifestations are another significant aspect of Churg Strauss Syndrome. Patients may develop purpura, nodules, or other dermatological lesions. These skin symptoms can be painful and may leave lasting scars.
Neurological Symptoms
Neurological involvement is a critical component of the syndrome. Patients may experience peripheral neuropathy or central nervous system involvement, both of which can have significant implications for patient management.
Peripheral Neuropathy
Peripheral neuropathy is a common neurological manifestation, often presenting as mononeuritis multiplex. This can result in significant morbidity, including muscle weakness and sensory loss.
Central Nervous System Involvement
Central nervous system involvement can manifest as encephalopathy, seizures, or other serious neurological complications. Prompt recognition and treatment are critical to prevent long-term damage.
Organ System Involvement in Churg Strauss Syndrome
Churg Strauss Syndrome, also known as eosinophilic granulomatosis with polyangiitis, is a complex autoimmune condition. It is characterized by the presence of eosinophils and vasculitis. This condition significantly impacts various organ systems, affecting patient management and outcomes.
Cardiovascular Complications
Cardiovascular issues are a major cause of morbidity and mortality in Churg Strauss Syndrome patients. The heart and blood vessels can be affected in several ways, leading to severe consequences.
Myocarditis and Pericarditis
Myocarditis, or inflammation of the heart muscle, and pericarditis, or inflammation of the heart sac, are common. These can cause heart failure, arrhythmias, and even death if not managed correctly.
Coronary Artery Involvement
Coronary artery involvement is another complication of Churg Strauss Syndrome. It can cause angina, myocardial infarction, and other coronary events.
Gastrointestinal Involvement
Gastrointestinal symptoms are prevalent in Churg Strauss Syndrome. They range from mild abdominal pain to severe gastrointestinal bleeding. Eosinophilic infiltration can lead to bowel obstruction and perforation.
Renal Manifestations
Renal involvement is a critical aspect of Churg Strauss Syndrome. It can have long-term effects on kidney function. Symptoms range from mild urinary issues to rapidly progressive glomerulonephritis, which can cause kidney failure if untreated.
Managing renal manifestations involves immunosuppressive therapy and monitoring kidney function. This is to prevent long-term damage.
Diagnostic Criteria and Evaluation
The diagnosis of Churg Strauss Syndrome involves a mix of clinical signs, lab results, and imaging. Getting it right is key to starting the right treatment and bettering patient outcomes.
American College of Rheumatology Criteria
In 1990, the American College of Rheumatology (ACR) set criteria for Churg Strauss Syndrome. A patient must meet at least four of the six criteria to be classified:
- Asthma
- Eosinophilia greater than 10%
- Mononeuropathy or polyneuropathy
- Non-fixed pulmonary infiltrates
- Paranasal sinus abnormality
- Extravascular eosinophils on biopsy
Laboratory Tests and Biomarkers
Laboratory tests are vital in diagnosing Churg Strauss Syndrome. Key tests include:
Blood Eosinophil Counts
Elevated blood eosinophil counts are a hallmark of the disease, showing up mainly during the eosinophilic phase.
ANCA Testing
Testing for anti-neutrophil cytoplasmic antibodies (ANCA) can help support the diagnosis. Yet, not all patients with Churg Strauss Syndrome test positive for ANCA.
Imaging Studies
Imaging studies are essential for assessing organ involvement and guiding management decisions.
Chest Radiography and CT Scans
Chest radiography and CT scans can reveal pulmonary infiltrates and other lung abnormalities.
Cardiac Imaging
Cardiac imaging, including echocardiography and MRI, is used to evaluate cardiac involvement and monitor for complications.
By combining these diagnostic approaches, clinicians can accurately diagnose Churg Strauss Syndrome. This leads to the development of an effective treatment plan.
Biopsy and Histopathological Findings
Biopsy is key in diagnosing Churg Strauss Syndrome, revealing tissue evidence of its hallmark features. This involves obtaining tissue samples for detailed histopathological examination.
Tissue Biopsy Techniques
Obtaining tissue samples through biopsy is essential for identifying disease-specific characteristics. The biopsy site selection depends on the organs affected and tissue accessibility.
Characteristic Histological Features
Churg Strauss Syndrome’s histopathological findings are distinct, featuring eosinophilic infiltration, granulomatous inflammation, and necrotizing vasculitis.
Eosinophilic Infiltration
Eosinophilic infiltration is a defining feature, where eosinophils gather in tissues, causing inflammation and damage.
Granulomatous Inflammation
Granulomatous inflammation results in the formation of granulomas. These are clusters of immune cells trying to contain inflammation.
Necrotizing Vasculitis
Necrotizing vasculitis involves inflammation of blood vessels, causing necrosis and damage to the vascular walls.
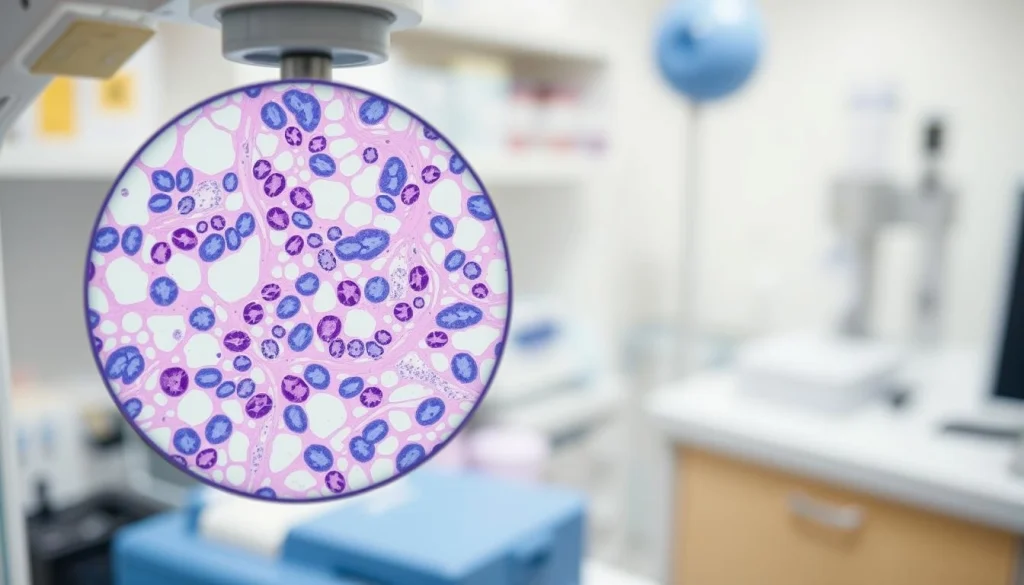
The combination of these histological features is critical for diagnosing Churg Strauss Syndrome. Biopsy and histopathological examination are essential for confirming the disease.
Differential Diagnosis
Diagnosing Churg Strauss Syndrome is complex due to its similarity with other vasculitides and hypereosinophilic syndromes. Accurate diagnosis is key for effective management and treatment.
Other Vasculitides
Distinguishing Churg Strauss Syndrome from other vasculitides is critical. Conditions like Wegener’s granulomatosis and microscopic polyangiitis share some clinical features. Yet, they have distinct characteristics that demand different treatment strategies.
Hypereosinophilic Syndromes
Hypereosinophilic syndromes, marked by high eosinophil counts, pose a diagnostic challenge. They are often confused with Churg Strauss Syndrome. Key conditions within this group include idiopathic hypereosinophilic syndrome and parasitic infections.
Idiopathic Hypereosinophilic Syndrome
Idiopathic hypereosinophilic syndrome is characterized by eosinophilia without a known cause. Distinguishing it from Churg Strauss Syndrome requires a detailed evaluation of clinical features and laboratory results.
Parasitic Infections
Certain parasitic infections can cause eosinophilia, mimicking Churg Strauss Syndrome. A thorough medical history and diagnostic tests are essential to exclude parasitic infections.
In conclusion, diagnosing Churg Strauss Syndrome requires a thorough evaluation to differentiate it from other vasculitides and hypereosinophilic syndromes. A precise diagnosis is essential for effective treatment and patient care.
Treatment Approaches for Churg Strauss Syndrome
Managing Churg Strauss Syndrome requires a multifaceted approach to reduce disease activity and enhance patient outcomes. The chosen treatment strategy hinges on the disease’s severity, the organs affected, and the patient’s health status.
Corticosteroid Therapy
Corticosteroids are the primary treatment for Churg Strauss Syndrome, focusing on acute inflammation and symptom control. They effectively lower eosinophil counts and mitigate vasculitic symptoms.
Immunosuppressive Medications
Immunosuppressive drugs complement corticosteroids in treating Churg Strauss Syndrome, essential when corticosteroids alone are insufficient or when organ involvement is significant.
Cyclophosphamide
Cyclophosphamide, a powerful immunosuppressant, is employed in severe Churg Strauss Syndrome cases, mainly when major organs are affected. It aids in achieving remission.
Azathioprine and Methotrexate
Azathioprine and methotrexate serve as immunosuppressive agents for maintaining remission in Churg Strauss Syndrome. They are considered for patients needing prolonged therapy.
Biologic Therapies
Biologic therapies represent a promising avenue for Churg Strauss Syndrome management, providing targeted disease treatment.
Mepolizumab
Mepolizumab, an anti-IL-5 antibody, has demonstrated efficacy in reducing asthma exacerbations and improving lung function in severe eosinophilic asthma, a common Churg Strauss Syndrome feature.
Rituximab
Rituximab, targeting CD20-positive B cells, has been applied in Churg Strauss Syndrome cases with notable vasculitic involvement.
Management of Specific Organ Involvement
Managing specific organ involvement is key in treating EGPA, significantly impacting patient outcomes. The disease affects various organs, requiring a customized treatment approach.
Pulmonary Management
Pulmonary involvement is a defining feature of EGPA, making symptom management critical. Treatment often includes corticosteroids to reduce inflammation and immunosuppressive therapy to control the disease. In severe cases, additional interventions may be needed to manage complications like asthma or pulmonary infiltrates.
Monitoring pulmonary function through tests like spirometry is essential. It helps assess for signs of asthma exacerbation. Adjusting treatment plans based on pulmonary involvement severity is vital for better patient outcomes.
Cardiac Management
Cardiac involvement in EGPA can be severe, leading to significant morbidity and mortality. Management aims to reduce inflammation and prevent cardiac damage. Corticosteroids are the first line of treatment, with immunosuppressive agents added in severe cases or with cardiac involvement.
Regular cardiac evaluations, including echocardiography and cardiac MRI, are critical. They help assess cardiac function and detect complications early. Managing cardiac involvement often requires a team effort, involving cardiologists and rheumatologists.
Neurological Management
Neurological manifestations of EGPA vary, from peripheral neuropathy to central nervous system involvement. Immunomodulatory therapy is used to control the disease. In severe cases, plasma exchange may be considered.
Neurological assessment and monitoring are vital. They help detect changes in neurological status and guide treatment adjustments. Collaboration between neurologists and rheumatologists is essential for effective management.
Prognosis and Long-term Outcomes
Understanding the prognosis and long-term outcomes for patients with Churg Strauss Syndrome is vital for effective management. The disease, now often referred to as Eosinophilic Granulomatosis with Polyangiitis (EGPA), has a varied prognosis. This depends on several factors.
Factors Affecting Prognosis
Several key factors influence the prognosis of EGPA patients. These include the severity of organ involvement and the patient’s response to initial treatment.
Five-Factor Score
The Five-Factor Score (FFS) is a critical tool used to assess prognosis. It considers factors such as renal insufficiency, proteinuria, cardiomyopathy, gastrointestinal involvement, and central nervous system involvement. A higher FFS indicates a poorer prognosis.
ANCA Status
The presence or absence of Anti-Neutrophil Cytoplasmic Antibodies (ANCA) also affects prognosis. ANCA-positive patients tend to have a different disease phenotype. They may respond differently to treatment compared to ANCA-negative patients.
Relapse Rates and Patterns
Relapse is a significant concern in EGPA, with rates varying based on treatment and patient factors. Understanding relapse patterns is essential for long-term management.
Studies have shown that relapse rates can be high, particular in patients with certain characteristics. These include ANCA positivity or a history of relapse. Monitoring and adjusting treatment strategies are critical for minimizing relapse risk.
Advances and Future Directions in Churg Strauss Syndrome Research
Research into Churg Strauss Syndrome, now more commonly referred to as Eosinophilic Granulomatosis with Polyangiitis (EGPA), is continually evolving. Recent advances in understanding the pathophysiology of EGPA have led to the development of novel therapeutic strategies.
One of the significant advances in EGPA research is the identification of specific biomarkers. These biomarkers aid in diagnosis and monitoring disease activity. Studies have shown that certain cytokines and eosinophil-related markers can predict disease relapse and treatment response.
Future directions in EGPA research include the exploration of targeted therapies. This includes biologics that target eosinophils or certain cytokines involved in the disease process. Ongoing clinical trials are investigating the efficacy and safety of these emerging treatments.
As our understanding of EGPA continues to grow, treatment approaches will likely become more personalized and effective. The integration of advances in genetics, immunology, and molecular biology will be key in shaping the future of EGPA management.
FAQ About Churg Strauss Syndrome
Q: What is Churg Strauss Syndrome?
A: Churg Strauss Syndrome, also known as Eosinophilic Granulomatosis with Polyangiitis (EGPA), is a rare autoimmune disorder. It is characterized by the presence of eosinophils, a type of white blood cell, and inflammation of blood vessels.
Q: What are the symptoms of Churg Strauss Syndrome?
A: Symptoms of Churg Strauss Syndrome vary. Common manifestations include respiratory symptoms like asthma and sinusitis. Skin manifestations and neurological symptoms such as peripheral neuropathy and central nervous system involvement are also common.
Q: How is Churg Strauss Syndrome diagnosed?
A: Diagnosis involves a combination of clinical criteria, laboratory tests, and imaging studies. Blood eosinophil counts and ANCA testing are key. Chest radiography, cardiac imaging, biopsy, and histopathological examination are also used.
Q: What are the treatment options for Churg Strauss Syndrome?
A: Treatment options include corticosteroid therapy and immunosuppressive medications like cyclophosphamide and azathioprine. Biologic therapies, including mepolizumab and rituximab, are also used. These aim to manage the condition and reduce disease activity.
Q: What is the prognosis for patients with Churg Strauss Syndrome?
A: Prognosis varies based on the Five-Factor Score and ANCA status. Some patients experience relapses. Long-term management is essential to improve outcomes.
Q: Can Churg Strauss Syndrome be cured?
A: While there is no cure, treatment can effectively manage the condition. It reduces symptoms and prevents complications, improving the quality of life for patients.
Q: How does Churg Strauss Syndrome affect different organ systems?
A: The condition can affect various organ systems. This includes the cardiovascular, gastrointestinal, and renal systems. Complications such as myocarditis, coronary artery involvement, and renal manifestations can occur.
Q: What is the role of biopsy in diagnosing Churg Strauss Syndrome?
A: Biopsy and histopathological examination are critical in confirming the diagnosis. They reveal characteristic features like eosinophilic infiltration, granulomatous inflammation, and necrotizing vasculitis.